
Use the table below to find the atomic weight of each atom (element), or refer to a Periodic Table of the Elements. Considering 37 as the maximum solubility of HCl, you can calculate the molarity using the solution density (1.2 g/mL) and HCls molar mass (36.46 g/mol).
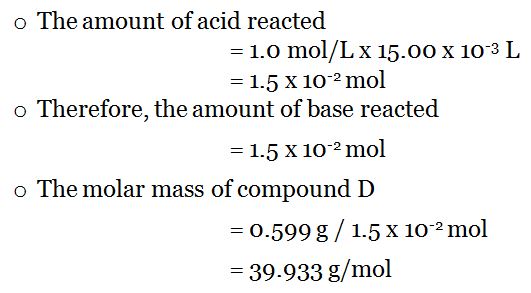
Molecular weight calculation: 1.00794 + 35. Let us calculate the molecular weight of some common compounds. The reagent used in laboratories is HCl dissolved in water, which is why youll find in the label that it is around 37 HCl in weight. Convert grams Hydrochloric Acid to moles or moles Hydrochloric Acid to grams. For example, in one mole of a chemical compound there are 6.022 x 1023 molecules. Relative molecular weight of HCl: 36.46094 Molar mass of HCl: 36.46094 g/mol (0.03646 kg/mol) MrHCl ArH + ArCl 1.00794 + 35.453 36.46094 Molar. One mole of 'something' contains 6.022 x 1023 entities. A mole is the unit that measures the amount of a substance. Molecular FormulaHCl Average mass36.461 Da Monoisotopic mass35.976677 Da. extreme kratom shot python read large file bisbee community cvt judder. The molality of a solution is defined as the. One thousand mers connected together would add up to a weight of 28,000 grams/mole and would have 6,000 atoms.Ī mole is the standard method in chemistry for communicating how much of a substance is present. ChemSpider 2D Image Hydrochloric acid HCl. Therefore, the number of moles of HCl that are present in 38g of HCl are mass of HCl / molar mass of HCl 38 / 36.5 1.041. Lets assume that you have 5 g of HCl in a 1.2 liter solution. Decide on the mass concentration of your substance you can either input it directly or fill in the boxes for substance mass and solution volume. We combine (react) many mers of ethylene together to form a polyethylene chain. For the hydrochloric acid, it is equal to 36.46 g/mol. The atomic weight of carbon is 12 and that of hydrogen is 1, so one mer of ethylene has a weight of 2(12) + 4(1) = 28. It has a total of 6 atoms: 2 carbon (C) atoms and 4 hydrogen (H) atoms. Hydrochloric Acid 36 -40 231-595-7 HCl 36 If the mass of the. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. DENSITY EXPANSION (DEX) APPROXIMATION FOR MIXTURE RDFs Molar mass of KOH 56.
#MOLAR MASS OF HCL HOW TO#


 0 kommentar(er)
0 kommentar(er)
